Equipe du Dr Cristine Alves Da Costa et du Dr Mounia Chami
Physiopathologie des Maladies d'ALZheimer et de PARKinson | Public
expert | Grand
public |
Activités de l'équipe
ALZPARK research aims the understanding of the physiopathological mechanisms of Alzheimer’s disease (AD) and Parkinson’s disease (PD). These two neurodegenerative pathologies are respectively defined by learning and memory defects linked to primary affection of the hippocampus in AD, and by movement disabilities associated with the degeneration of the dopaminergic nigrostriatal system in PD.
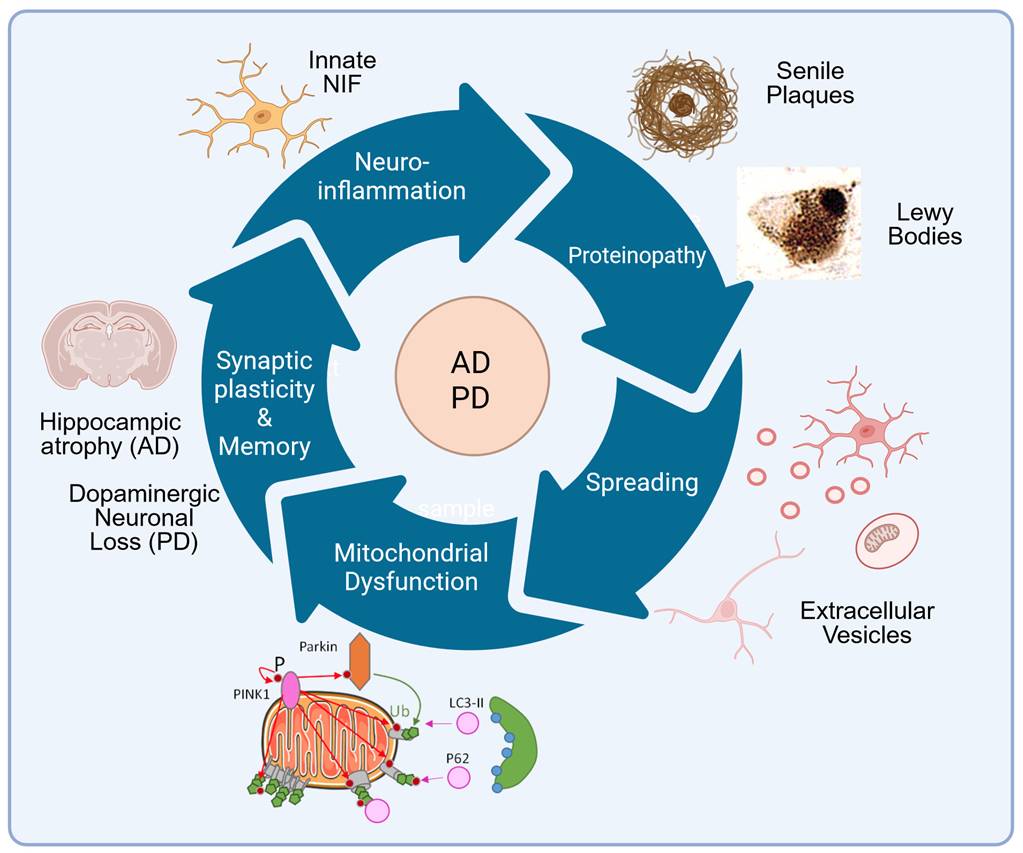
AD and PD share several key similarities including the accumulation of toxic/modified proteins, namely aggregates of hyperphosphorylated Tau protein in neurofibrillary tangles and of toxic amyloid beta (Aβ) peptides in senile plaques in AD; and aggregates of α-synuclein (α-Syn) in Lewy bodies in PD. These aggregates trigger an intracellular “proteinopathy” that spreads between the brain regions, as diseases progress. In addition, both AD and PD are multifactorial pathologies characterized by endolysosomal autophagic and mitochondrial dysfunctions, altered central innate neuroinflammation (NIF) and cognitive deficits.
However, the specific and/or common molecular mechanisms underlying these dysfunctions remain to be established.ALZPARK research will address four original axis to study:
I. The specific or common molecular mechanisms underlying mitochondria dysfuntion in AD & PD
II. Mechanisms controlling innate neuroinflammation in AD & PD
III. Mechanisms underlying cellular proteinopathy & pathological spreading in AD & PD
IV. Parkin as a molecular denominator between AD & PD
ALZPARK research develops using specific AD and PD models spanning from available in vitro cellular models, ex vivo organotypic hippocampal slice cultures, in vivo preclinical mice models (i.e. transgenic, KO and humanized KI, or by viral/pharmacological interventions).
ALZPARK research is developed at the interface between fundamental and clinical research by:
I. studying human-derived samples (human-derived brains) and cells (fibroblasts, PBMC and iPSC) in AD and PD .
Eysert et al. bioRxiv, 2023,.12.21.570579, doi: https://doi.org/10.1101/2023.12.21.570579
Xicota L. et al. Transl. Psychiatry, 2023 Feb 14;13(1):54
II. Investigating the role of Parkin as a molecular denominator between PD & brain tumors
Rouland L. et al. Theranostics 2021 Nov 1;11(20):10047-10063
Viotti J. et al. Oncogene 2014 Apr 3;33(14):1764-75
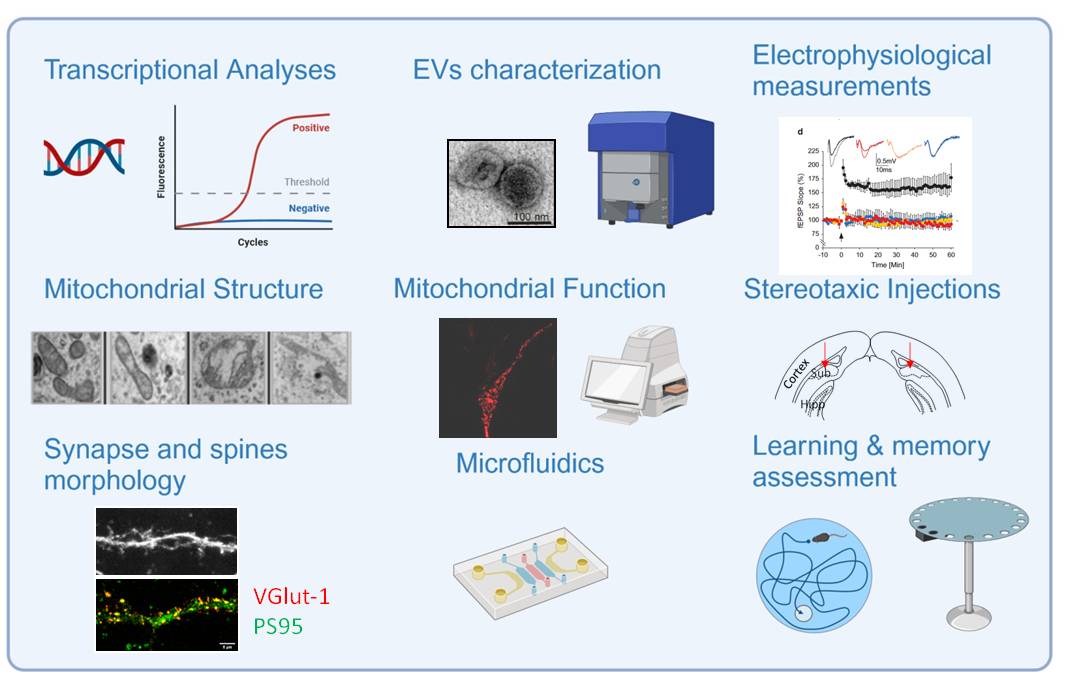
ALZPARK members have outstanding expertise in AD and PD and show complementary know-how including molecular/cellular biology, biochemistry, high resolution imagery, mitochondrial structure and function, transcriptional analysis and xCELLigence technology. The team has also expertise in behavioral studies in mice and recently developed AAV-mice models. To study the spreading, they also set-up new approaches including secretome analysis and EVs preparation and their biochemical and functional characterization. The project includes systematic RNA seq, lipidomic and proteomic analyses, scRNA seq and spatial omics technologies and bioinformatic analysis. The team recently set up protocols for the analyses of mitochondria function and NIF on brain dissociated single cells using cytometry and developed compartmentalized microfluidic technology.
 Key recent publications
Key recent publications :
Lauritzen, I., et al. Acta Neuropathol, 2016. 132(2): p. 257-276.
Lacampagne, A et al. Acta Neuropathologica, 2017.
Bourgeois, A., et al. Neurobiol Aging, 2018. 71: p. 21-31.
Vaillant-Beuchot, L., et al. Acta Neuropathol, 2021. 141(1): p. 39-65.
Goiran, T., et al., Cell Death Differ, 2018. 25(5): p. 873-884.
Duplan, E., et al. Molecular Neurodegeneration 2016
El Manaa, W., et al. Autophagy, 2021: p. 1-23.
